


Minoxidil is a pyrimidine derivative originally developed as an oral antihypertensive agent. It is now widely used in topical formulations to treat androgenetic alopecia and other forms of hair loss. Minoxidil acts as a potassium channel opener, leading to vasodilation and improved blood flow to hair follicles, thereby stimulating hair growth. The CAS number 83701-22-8 refers to Minoxidil sulfate, the active metabolite of Minoxidil, which is formed in the body and is also involved in promoting hair growth.


Minoxidil is a pyrimidine derivative originally developed as an oral antihypertensive agent. It is now widely used in topical formulations to treat androgenetic alopecia and other forms of hair loss. Minoxidil acts as a potassium channel opener, leading to vasodilation and improved blood flow to hair follicles, thereby stimulating hair growth. The CAS number 83701-22-8 refers to Minoxidil sulfate, the active metabolite of Minoxidil, which is formed in the body and is also involved in promoting hair growth.

.3d8f8f41.svg)
Pharmaceutical
.3556d45a.svg)

Pharmaceutical Actives & Precursors


Active Pharmaceutical Ingredients (APIs)
Included in Quote
Included in Quote
Included in Quote
Included in Quote
.7767eb0f.png)

Chemical Properties & Specifications
Topical Minoxidil is applied to the scalp to promote hair regrowth in individuals with androgenetic alopecia.
Oral Minoxidil is used as a vasodilator to treat severe hypertension.
Minoxidil is included in various cosmetic formulations aimed at enhancing hair density and thickness.
Minoxidil acts as a potassium channel opener, which allows more potassium ions to flow out of hair follicles. This increases blood flow to the hair follicle, stimulating hair growth and improving the quality of existing hair.
Minoxidil is primarily used in the pharmaceutical industry for treating androgenetic alopecia (male and female pattern baldness). It is formulated into topical solutions and foams for application to the scalp. Additionally, it is utilized in the development of hair loss treatment medications.
Yes, Minoxidil is considered safe for long-term use when used as directed. Topical formulations have minimal systemic absorption, making it safe for continuous use. However, discontinuation may lead to hair loss resumption.
Yes, Minoxidil is FDA-approved for both men and women, though the formulations for each gender may vary in concentration. Women typically use a 2% solution or a 5% foam, while men commonly use the 5% solution.
Most users begin to see noticeable hair growth after about 2 to 4 months of consistent use, though individual results may vary. It’s important to continue using the product to maintain benefits.
Common side effects of Minoxidil include scalp irritation, itching, dryness, and flaking. Rare side effects may include dizziness, chest pain, and rapid heartbeats. Always consult your doctor if you experience severe side effects.
Yes, Minoxidil can be used in combination with other treatments like finasteride (an oral medication for hair loss). However, it is important to consult a healthcare provider before combining treatments to avoid potential interactions.

CAS No. : 97952-72-2
Category : Nutraceutical Ingredients
Sub-Category : Herbal Extracts
Description: Boswellia Serrata Extract standardized to 65% boswellic acids is a potent anti-inflammatory herbal e...

CAS No. : 13185-33-4
Category : Nutraceutical Ingredients
Sub-Category : Mineral Extracts
Description: Shuddha Shilajit is a purified and concentrated dry extract derived from natural exudates found in h...
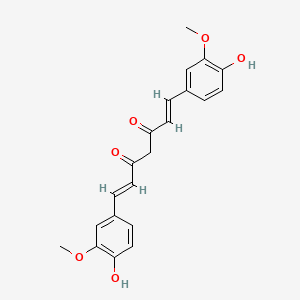
CAS No. : 458-37-7
Category : Nutraceutical Ingredients
Sub-Category : Plant Extracts
Description: Curcumin 95% is a standardized turmeric extract derived from the rhizomes of Curcuma longa, enriched...

CAS No. : 90147-43-6
Category : Nutraceutical Ingredients
Sub-Category : Herbal Extracts
Description: Ashwagandha Extract standardized to 5.0% withanolides is a high-strength adaptogen sourced from the ...
